-
Feed de Notícias
- EXPLORAR
-
Páginas
-
Blogs
-
Courses
-
Movies
Recombinant Trypsin Powder Market to Hit USD 126.23 Million by 2032, Says Introspective Market Research
Recombinant Trypsin Powder Market to Hit USD 126.23 Million by 2032, Says
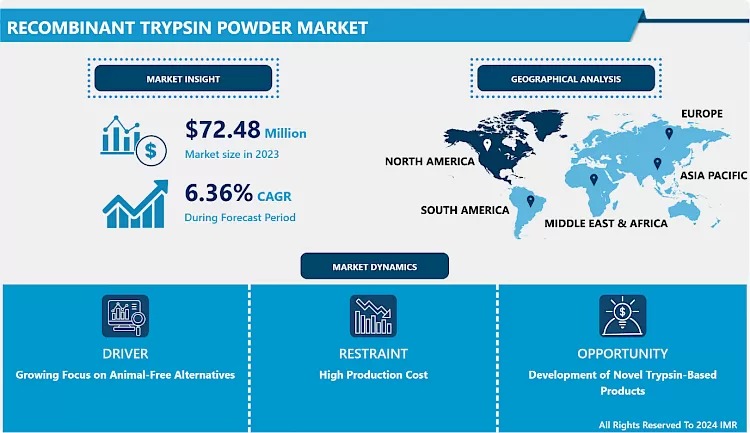
According to a new report from, the Global Recombinant Trypsin Powder Market was valued at USD 72.48 million in 2023 and is forecast to reach USD 126.23 million by 2032, growing at a compound annual growth rate (CAGR) of 6.36% over the 2024–2032 period.
This robust growth is being fueled by increasing adoption in biopharmaceutical manufacturing, cutting-edge proteomics, and cell and tissue culture. Recombinant trypsin, produced via genetic engineering in microorganisms such as E. coli or yeast, offers unmatched purity and safety compared to animal-derived alternatives — making it an essential reagent in next-generation biologics production.
Quick Insights
- 2023 Market Size: USD 72.48 million
- 2032 Forecast: USD 126.23 million
- Forecast CAGR (2024–2032): 6.36%
- Primary Applications: Biopharmaceutical manufacturing, cell culture / tissue engineering, proteomics, therapeutics
- Leading End Users: Pharma companies, biotech firms, academic & research institutes
- Top Source Technologies: Microbial-based (e.g., bacterial), yeast-based recombinant trypsin
- Key Players (highlighted in comparable markets): Novozymes, Thermo Fisher Scientific, Merck KGaA, Sartorius, Yaxin Biotechnology, Yocon Hengye Bio, BasalMedia, Pu Tai Bio
What’s Driving the Growth?
Biopharma Moves Away from Animal-Derived Enzymes
Regulatory and ethical pressures are pushing biomanufacturers toward animal-free processes. Recombinant trypsin—produced in controlled microbial systems—reduces the risk of animal-borne contaminants and increases batch consistency.
Cell Culture & Regenerative Medicine Boom
As cell-therapy, stem-cell research, and tissue engineering accelerate, demand for high-purity, recombinant-grade trypsin is rising. Its gentle yet effective activity makes it ideal for detaching adherent cells while minimizing damage.
Proteomics & Research Applications
Recombinant trypsin is also a cornerstone for proteomic workflows — being used to digest proteins before mass spectrometry, enabling more accurate, consistent results.
Packaging & Stability Advantages
Powder formulation enhances shelf life, simplifies shipping, and improves storage flexibility for labs and manufacturing setups.
Where Are the Opportunities & Emerging Trends?
Could microbial- or yeast-derived trypsin revolutionize enzyme sourcing in bioprocessing?
- Microbial-based recombinant systems offer cost-efficiencies and scalability. As process optimization improves, their cost profile could undercut traditional sources.
- Yeast-derived trypsin is gaining traction for being animal-component-free while offering robust activity.
Will the surge in cell therapy fuel a new wave of demand?
- As more companies invest in cell-based therapies, the need for reliable, GMP-grade trypsin for scale-up will only grow.
- There's potential for custom formulations tailored to sensitive cell types (e.g., iPSCs, stem cells).
Could sustainability and regulatory pressures accelerate adoption?
- Clean-label demands and biosafety concerns are encouraging a shift away from animal-derived reagents.
- Regulatory bodies may increasingly favor reagents with traceable, recombinant origins for clinical-grade manufacturing.
Expert Voice
“Recombinant trypsin powder is rapidly becoming a non-negotiable reagent in modern biologics manufacturing,” said Dr. Priya Nair, Principal Consultant, Life Sciences, at. “The consistent quality, safety profile, and scalability of recombinant systems directly address the challenges posed by animal-derived trypsin. As bioprocessing advances, especially in regenerative medicine and cell therapy, we expect this market to not just grow—but transform the way enzymes are sourced and used.”
Regional & Segment Outlook
- North America continues to dominate due to a strong biotech ecosystem, advanced regulatory infrastructure, and widespread adoption of recombinant reagents.
- Europe follows, leveraging stringent safety standards and sustainability goals to drive uptake.
- Asia-Pacific is expected to see the fastest growth, powered by rising biopharma investments in China and India, expanding research infrastructure, and increasing domestic manufacturing of therapeutic proteins.
- By Application, biopharmaceutical manufacturing holds the largest share, but cell culture and proteomics are fastest growing.
- By Source, microbial-derived recombinant trypsin leads, followed closely by yeast-based variants.
Breakthroughs & Innovation Highlights
- Engineered microbial platforms: Several firms are engineering microbes (e.g., E. coli) to express high-purity trypsin with lower autolysis rates, improving yield and stability.
- GMP-grade formulations: Leading players are rolling out GMP-compliant recombinant trypsin for use in clinical and commercial biomanufacturing.
- Lyophilization advances: New freeze-drying technologies are enhancing the stability and shelf life of trypsin powder without compromising activity.
Challenges to Watch
- Production Cost Pressures: Despite its advantages, recombinant trypsin is still costlier than traditional animal-derived enzymes, making it less accessible to smaller labs and price-sensitive markets.
- Autolysis & Stability: Trypsin’s inherent self-digestion (autolysis) remains a technical challenge, potentially reducing shelf life or activity during storage.
- Regulatory Burden: Scaling production to GMP-grade levels demands strict quality controls, traceability, and regulatory compliance, increasing barriers to entry.
- Competition from Alternatives: Other proteases (e.g., enterokinase, collagenase) or enzyme-free dissociation chemicals could displace trypsin in some workflows.
Use-Case / Case Study
Case Study – Cell Therapy Startup
A mid-sized cell therapy company developing a CAR-T therapy replaced its animal-derived trypsin with recombinant trypsin powder. The result? Improved cell viability during harvesting, reduced lot-to-lot variability, and a streamlined supply chain that supports future GMP batch scale-up. This switch not only improved process robustness but also aligned with their commitment to animal-free manufacturing.
About
Introspective Market Research (IMR) is a leading global insights firm specializing in biotechnology, healthcare, and specialty ingredients. With deep domain expertise and rigorous data methodology, IMR helps companies make well-grounded strategic decisions by delivering forward-looking market intelligence, competitive benchmarking, and scenario planning.
Call to Action
To learn more or to download a free sample of our Recombinant Trypsin Powder Market Report, please visit:
Download Sample Report
You can also schedule a one-on-one briefing with one of our Principal Consultants to discuss customized insights for your business—contact us at +91-74101-03736 or sales@introspectivemarketresearch.com.
Contact
Introspective Market Research
Phone: +91-74101-03736 | +91-95790-51919
Email: sales@introspectivemarketresearch.com
Website: introspectivemarketresearch.com
- Recombinant_Trypsin_Powder_Market
- Recombinant_Trypsin_Market_Report
- Trypsin_Powder_Industry_Analysis
- Biopharmaceutical_Enzymes_Market
- Cell_Culture_Reagents_Market
- Proteomics_Enzyme_Market
- Animal-Free_Trypsin_Market
- Biotechnology_Enzymes_Market
- Recombinant_Enzymes_Industry
- IMR_Market_Research
- Introspective_Market_Research_Press_Release
- Bioprocessing_Enzymes_Market
- GMP_Grade_Trypsin
- Microbial_Derived_Trypsin
- Yeast_Derived_Trypsin
- Biotech_Industry_Trends_2025
- Enzyme_Manufacturing_Market
- Life_Sciences_Market_Insights
- Global_Trypsin_Market_Forecast
- Biotech_Reagents_Market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


