-
Feed de Notícias
- EXPLORAR
-
Páginas
-
Blogs
-
Courses
-
Movies
Osteogenesis Imperfecta Treatment Market - Trend, Growth, Forecast 2024–2032
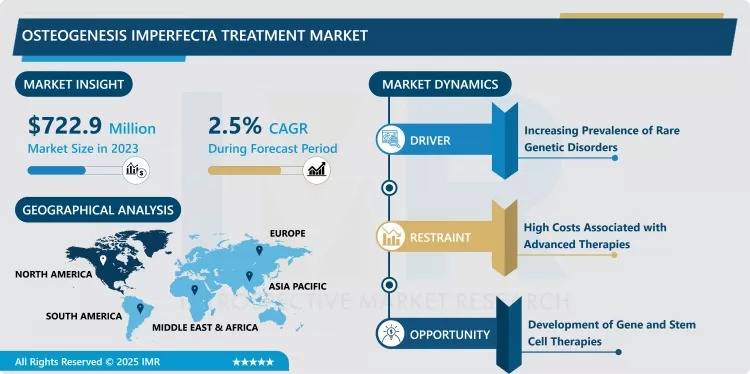
Introspective Market Research (IMR) has unveiled its latest strategic analysis on the Global Osteogenesis Imperfecta (OI) Treatment Market. The report highlights the critical role of specialized pharmaceuticals and emerging gene therapies in managing this rare, inherited connective tissue disorder, often referred to as "brittle bone disease." The market, which was valued at USD 722.9 Million in 2023, is projected to expand to USD 902.80 Million by 2032, demonstrating a Compound Annual Growth Rate (CAGR) of 2.5% over the forecast period of 2024–2032.
While the CAGR reflects the specialized, high-cost nature and limited patient pool of an orphan drug market, the growth is qualitatively significant, underpinned by advancements that are fundamentally shifting patient management from palliative care to disease modification. The primary drivers are the increasing global prevalence of rare genetic disorders, enhanced diagnostic capabilities (especially genetic testing), and robust R&D pipelines focused on next-generation biologics and curative gene therapies.
Quick Insights: The Osteogenesis Imperfecta Treatment Market Snapshot
- Market Valuation (2023): USD 722.9 Million
- Projected Market Valuation (2032): USD 902.80 Million
- Growth Rate (CAGR 2024–2032): 2.5%
- Key Market Driver: Increasing global focus and funding for rare genetic disorders, leading to earlier and more accurate diagnosis.
- Key Market Opportunity: Development and commercialization of Gene and Stem Cell Therapies aimed at correcting the underlying collagen defect.
- Leading Drug Segment: Denosumab (A biologic therapy expected to dominate due to its high efficacy in increasing bone density and reducing fracture rates by inhibiting bone resorption).
- Leading Route of Administration: Intravenous (IV) (Preferred for administering high-dose bisphosphonates and biologics, ensuring higher bioavailability and compliance for severe cases).
- Dominant Regional Market: North America (Holds the largest share due to strong healthcare infrastructure, well-defined reimbursement pathways for orphan drugs, and high R&D investment).
- Top Industry Players: Amgen Inc., Pfizer Inc., BioMarin Pharmaceutical Inc., Ultragenyx Pharmaceutical Inc., and Takeda Pharmaceutical Company Limited.
Osteogenesis Imperfecta Treatment Market Segment Breakdown: Denosumab and IV Dominance
|
Segment Category |
Leading Sub-Segment |
Key Growth Driver |
|
By Drugs |
Denosumab |
Highly selective mechanism (RANK ligand inhibitor) offering superior anti-resorptive effects compared to traditional bisphosphonates. |
|
By Route of Administration |
Intravenous |
Ensures maximum drug compliance and bioavailability, essential for effective treatment in severe OI types (Pamidronate, Zoledronic Acid, and some biologics). |
|
By Region |
North America |
Favorable regulatory environment (Orphan Drug Designation) and extensive insurance coverage for high-cost rare disease treatments. |
Is Gene Therapy Poised to Offer a Functional Cure, and How are Biologics Bridging the Gap?
The treatment paradigm for Osteogenesis Imperfecta is undergoing a significant transformation, moving beyond the standard of care—intravenous bisphosphonates—toward highly targeted biologics and potentially curative gene therapies.
A major trend involves the rising adoption of biologics, such as Denosumab and the investigational Setrusumab (a monoclonal antibody targeting sclerostin). Unlike older therapies that slow bone breakdown, these biologics offer a more sophisticated mechanism, specifically modulating the bone remodeling process to improve mineralization and reduce fracture incidence. Denosumab, in particular, is gaining traction due to robust clinical data demonstrating its ability to significantly increase bone mineral density across pediatric and adult patient populations.
The ultimate opportunity lies in Gene and Stem Cell Therapies. This research focuses on delivering a functional copy of the defective collagen-producing gene (often COL1A1 or COL1A2) or administering mesenchymal stem cells that can differentiate into healthy osteoblasts. While still in early phases, this approach promises to correct the underlying molecular defect, transforming OI from a chronic condition into a potentially curable one. Industry collaborations between academic institutions and biotechnology firms are intensifying to accelerate these platforms into Phase 2 and 3 trials.
Expert Insight on the Future of OI Treatment
“For a long time, the management of Osteogenesis Imperfecta was limited to minimizing symptoms and maximizing mobility. Today, that is changing. The market is not seeing rapid growth in patient volume, but rather explosive growth in therapeutic value. The move to advanced biologics like setrusumab, which targets sclerostin to promote bone formation, marks a monumental shift from purely anti-resorptive to anabolic (bone-building) treatments. This qualitative leap is key. However, the commercial success of these novel drugs is intrinsically linked to early diagnosis via genetic sequencing and the continued availability of patient assistance programs to navigate the extremely high price points—a necessity for all orphan drugs. The success of the Phase 3 trials currently underway will be the most decisive factor in determining the market’s structure for the next decade.”
— Dr. Eleanor Voss, Principal Consultant, Rare Diseases & Genetic Therapeutics, Introspective Market Research
Latest Breakthroughs: Ultragenyx's Anabolic Pipeline
A notable recent breakthrough was announced in July 2023 by Ultragenyx Pharmaceutical Inc., which initiated Phase 3 clinical trials for its investigational monoclonal antibody, setrusumab (UX143). The trials—Orbit (for patients aged 5 to <26) and Cosmic (for patients aged 2 to <5)—are strategically designed to evaluate the drug's impact on the annualized clinical fracture rate compared to both placebo and current intravenous bisphosphonate therapy. This focus on an anabolic mechanism, promoting new bone growth, is considered a significant innovation in addressing the core pathology of OI Types I, III, and IV.
Challenges: The Rare Disease Economic Model and Cost Barriers
Despite the clinical advancements, the primary restraint remains the high costs associated with advanced therapies. As a rare (orphan) disease, drug development faces smaller patient populations, necessitating higher unit pricing to recoup significant R&D investments. Furthermore, treatment often involves long-term, specialized care delivered in hospital settings, contributing to overall high healthcare expenditure. For many patients globally, securing adequate reimbursement and overcoming socioeconomic barriers to access the gold-standard intravenous or biologic therapies remains a formidable challenge that the market players and regulatory bodies must continue to address.
Case Study: The Impact of Early Intervention with Denosumab
A 12-year-old female diagnosed with Type IV OI was experiencing an average of three long-bone fractures per year, severely limiting her mobility and development. Traditional oral bisphosphonates provided minimal relief.
The Solution: The patient was transitioned to Denosumab therapy, administered subcutaneously every six months, alongside intensive physical therapy. The treatment was covered under a specialized rare disease patient support program.
The Outcome: Following 24 months of treatment, the patient experienced a substantial increase in Lumbar Spine Bone Mineral Density (BMD z-score) by +1.2 standard deviations. Crucially, her fracture rate decreased from three per year to zero. This case exemplifies the power of targeted biologic intervention in stabilizing skeletal integrity, improving mobility, and drastically enhancing a pediatric patient's quality of life.
About Introspective Market Research
Introspective Market Research is a premier global market research firm specializing in detailed analysis and strategic advisory services across critical healthcare segments, including rare diseases, pharmaceuticals, and biotechnology. Our mission is to provide clients with the data-driven foresight necessary to navigate complex market dynamics and capitalize on emerging growth opportunities.
Call to Action: To gain a detailed competitive analysis of the Denosumab and Setrusumab pipelines, obtain the latest clinical trial data, or analyze granular segmentation by OI type and country, request the full report or schedule a strategic briefing with our rare disease analysts.
Download Complimentary Sample Report
Contact: Introspective Market Research
Sales Inquiries: sales@introspectivemarketresearch.com
Phone: +1 773 382 1049 (US)
- Osteogenesis_Imperfecta_Treatment_Market
- OI_Treatment
- Rare_Disease_Market
- Orphan_Drugs
- Biologics
- Gene_Therapy
- Setrusumab
- Denosumab
- Intravenous_Administration
- Bisphosphonates
- Anabolic_Therapeutics
- Bone_Mineral_Density
- Fracture_Rate
- Ultragenyx_Pharmaceutical_Inc.
- Amgen_Inc.
- Pfizer_Inc.
- North_America_Market
- Genetic_Disorders
- Market_Forecast
- Healthcare_Technology.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


