-
Fil d’actualités
- EXPLORER
-
Pages
-
Blogs
-
Courses
-
Film
Leptospirosis Market Forecast to Reach USD 810.93 million by 2032-IMR Report.
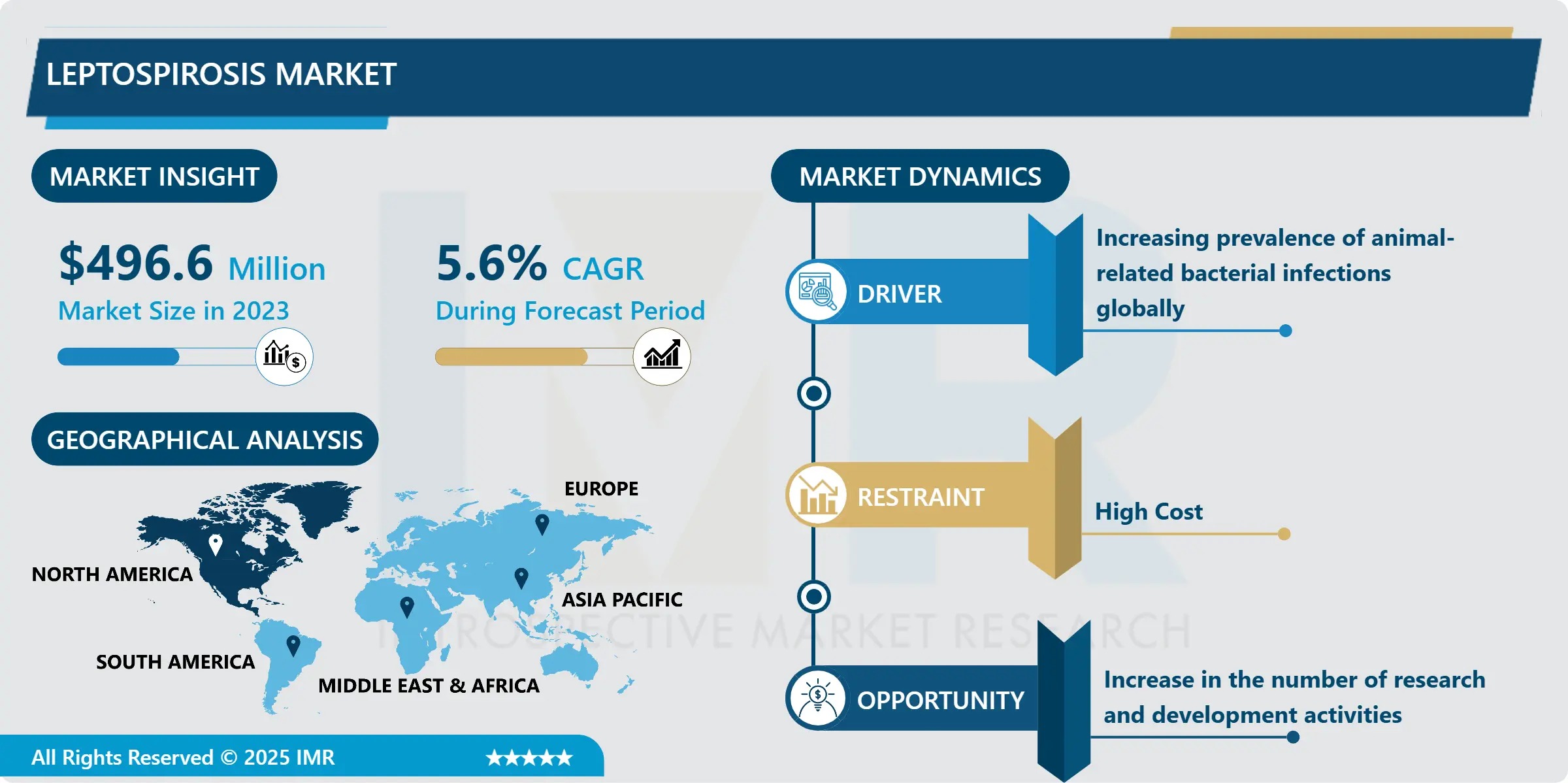
The Global Leptospirosis Market, valued at 496.6 million in 2023, and is Projected to Reach USD 810.93 million by 2032, Growing at a CAGR of 5.6% From 2024-2032, according to a new in-depth analysis by Introspective Market Research. Leptospirosis-a re-emerging zoonotic disease caused by pathogenic Leptospira spp.-s gaining renewed attention from global health authorities as climate change intensifies flooding events, urban rodent infestations, and agricultural exposure risks across tropical and subtropical regions.
This growth is propelled by expanding diagnostic accessibility in low-resource settings, growing inclusion of leptospirosis in national One Health surveillance frameworks, and rising public–private investment in next-generation vaccines and rapid point-of-care (POC) tests. While historically underdiagnosed and misclassified as dengue or malaria, leptospirosis is now being systematically integrated into differential testing algorithms-especially in Southeast Asia, Latin America, and Sub-Saharan Africa-unlocking new demand for serological assays, molecular kits, and prophylactic solutions.
Quick Insights: Leptospirosis Market at a Glance
- 2024 Market Size: USD 580.32 Million
- 2035 Projected Value: USD 1.24 Billion
- CAGR (2025–2035): 7.25%
- Fastest-Growing Segment: Diagnostics (especially rapid IgM/IgG lateral flow assays and multiplex PCR panels)
- Dominant Regional Market: Asia Pacific (34.18% share in 2024; expected to remain #1 through 2035)
- Top End-Use Settings: Hospitals & Clinics (42%), Reference Laboratories (28%), Public Health Programs (19%)
- Key Players: bioMérieux SA, Abbott Laboratories, Thermo Fisher Scientific, QIAGEN N.V., SD Biosensor (Standard Diagnostics), Zhejiang Orient Gene Biotech, Fortress Diagnostics, InBios International, Hangzhou Biotest Biotech, Creative Diagnostics
- Therapeutic Pipeline Highlights: 3 recombinant subunit vaccines (Phase I/II), 2 live-attenuated candidates, and 1 mRNA-based preclinical candidate as of Q3 2025
Why Is Leptospirosis Emerging from the Shadows of Neglect—and What Does That Mean for Global Health Infrastructure?
Once relegated to the periphery of tropical medicine, leptospirosis is now at the forefront of climate-resilient health planning. The bacteria thrive in warm, wet environments-particularly floodwaters contaminated with rodent or livestock urine-and outbreaks are increasingly triggered by extreme weather events. In 2024 alone, post-monsoon surges in India, typhoon-linked cases in the Philippines, and unprecedented urban outbreaks in São Paulo underscored the disease’s expanding epidemiological footprint.
Unlike malaria or dengue, leptospirosis lacks a WHO-prequalified rapid test-creating a critical diagnostic gap. But this is changing: new-generation assays are being validated for field deployment under the WHO ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid, Equipment-free, Deliverable), while countries like Thailand and Sri Lanka have begun integrating leptospirosis screening into fever surveillance networks.
“Leptospirosis isn’t just a ‘farmer’s disease’ anymore—it’s an urban, occupational, and climate-sensitive threat that crosses socioeconomic lines,” says Dr. Nandini Rao, Principal Consultant, Infectious Disease Diagnostics Practice at Introspective Market Research.
“What we’re seeing is a structural shift: Ministries of Health are no longer reacting to outbreaks—they’re embedding leptospirosis into predictive surveillance. That means routine testing in flood-response kits, inclusion in multiplex fever panels, and pre-emptive serosurveys in high-risk districts. The market opportunity lies not just in selling more tests—but in enabling diagnostic ecosystems that reduce case fatality from 10–15% to under 3%.”
Regional Deep Dive: Where the Burden-and Opportunity-Reside
Asia Pacific (34.18% Market Share)
APAC remains the epicenter of both disease burden and market activity. India reports over 15,000 confirmed cases annually (with estimated underreporting at 80%), concentrated in Kerala, Maharashtra, and Odisha. Thailand’s national leptospirosis control program—featuring free doxycycline prophylaxis for rice farmers during rainy season—has reduced incidence by 40% since 2020 and catalyzed local test kit manufacturing.
Indonesia, the Philippines, and Vietnam are investing in mobile diagnostic units for remote island communities, while China is scaling up Leptospira culture-free qPCR adoption in tier-2 hospitals. Japan and South Korea, though low-incidence, lead in R&D-hosting two of the three active Phase II vaccine trials.
Latin America (28.6% Share)
Brazil accounts for ~60% of regional cases, with outbreaks increasingly reported in urban slums (favelas) where open sewage and rodent density intersect. The Brazilian Ministry of Health now mandates differential testing for leptospirosis in all suspected dengue cases during high-rainfall months-a policy that boosted test volumes by 220% in 2024.
In Peru and Colombia, mining and sugarcane industries are funding worksite health programs that include baseline serology and prophylaxis-creating B2B demand for occupational health diagnostics.
Africa & Middle East (16.3% Share)
While reporting remains fragmented, seroprevalence studies suggest high exposure in Egypt (Nile Delta agriculture), Kenya (flood-prone informal settlements), and Madagascar (post-cyclone outbreaks). The Africa CDC’s Pathogen Genomics Initiative now includes Leptospira in its priority pathogen list-paving the way for regional assay harmonization and bulk procurement.
Segmentation Breakdown: Diagnostics Dominate, But Prevention Is the Future
By Product Type (2024–2032 Outlook)
|
Segment
|
2024 Share
|
Key Growth Catalysts
|
|---|---|---|
|
Rapid Diagnostic Tests (RDTs)
|
~38%
|
Low-cost, no-instrument use; WHO prequalification pathway underway (2026 target); ideal for community health workers
|
|
ELISA Kits
|
~26%
|
High-throughput screening in reference labs; rising automation in APAC public labs
|
|
Molecular Diagnostics (PCR, qPCR, LAMP)
|
~22%
|
Gold standard sensitivity; integration into syndromic fever panels (e.g., with dengue, chikungunya, malaria); price decline of 15–20% since 2022
|
|
Culture & Dark-Field Microscopy
|
<6%
|
Declining due to low sensitivity (<50%) and 7–14 day turnaround
|
|
Vaccines & Prophylaxis (Pipeline)
|
~8% (projected by 2030)
|
No licensed human vaccine yet—but strong momentum in veterinary (cattle, swine) and emerging human candidates
|
By End User
- Hospitals & Clinics: Largest segment (42%)-driven by improved clinical awareness and inclusion in fever workups
- Reference & Diagnostic Labs: 28%-growth fueled by national surveillance programs and cross-border sample referral networks
- Public Health Agencies & NGOs: 19%-expanding procurement for outbreak response, serosurveys, and occupational screening
- Research & Academic Institutions: 11%-focused on strain typing, antimicrobial resistance monitoring, and vaccine development
Innovation Spotlight: Who’s Shaping the Next Generation of Leptospirosis Management?
- bioMérieux (France): Launched LeptoPLEX™ in Q2 2025—a CE-marked multiplex PCR panel that detects 12 pathogenic Leptospira serovars in <90 minutes, with built-in inhibition controls. Now deployed in Thailand’s National Institute of Health network.
- SD Biosensor (South Korea): Partnered with FIND and the Thai MOH to field-validate Standard™ Lepto IgM/IgG Duo—a dual-marker lateral flow test with 94.2% sensitivity at day 5+ post-onset (vs. 78% for legacy RDTs). Rollout expected across ASEAN by 2026.
- Zhejiang Orient Gene Biotech (China): Secured WHO Emergency Use Listing (EUL) for its LeptoRapid® cassette in September 2025—the first leptospirosis RDT to meet all ASSURED+ criteria, priced at USD 1.85/unit in bulk.
- InBios International (USA): Advanced its LeptoChek® ELISA to detect early-phase antibodies (IgM + IgA) for improved day-3 sensitivity; now included in Brazil’s SUS-approved test formulary.
Cost-Efficiency & Access Strategies Gaining Traction
- Multiplexing: Bundling leptospirosis into syndromic panels reduces per-pathogen cost by 35–50% and improves differential diagnosis accuracy.
- Local Assembly & Tech Transfer: Partnerships like SD Biosensor–BioNet-Asia enable regional kit assembly in Thailand-cutting import duties and cold-chain logistics.
- Prophylaxis-as-Prevention: Doxycycline mass drug administration (MDA) in high-risk occupational groups remains the most cost-effective strategy (ICER: USD 42/DALY averted vs. USD 210 for hospital-based case management).
- Digital Integration: mHealth apps (e.g., India’s LeptoTrack) geotag suspected cases in real time—enabling targeted vector control and test-kit prepositioning.
Benefits Across the Ecosystem
- For Clinicians: Earlier, more accurate diagnosis reduces mismanagement (e.g., inappropriate antimalarials) and severe complications (Weil’s disease, AKI)
- For Public Health Systems: Real-time data feeds outbreak forecasting models and justifies resource allocation (e.g., flood kits, antibiotic stockpiles)
- For Manufacturers: Entry into WHO/Global Fund procurement pipelines via prequalification pathways
- For Communities: Community-led testing and education reduce stigma and improve help-seeking behavior
Access the Full Strategic Intelligence Report
The Leptospirosis Market Report (2024–2032) by Introspective Market Research delivers 220+ pages of actionable insights, including:
- Country-level prevalence maps, testing coverage gaps, and reimbursement landscapes
- Diagnostic assay performance benchmarking (sensitivity/specificity, LoD, turnaround time)
- Regulatory pathway analysis: FDA Breakthrough Device, CE-IVDR, WHO EUL, India CDSCO
- Pipeline tracker: 12 vaccines, 8 novel RDTs, 5 AI-enabled image analysis tools in development
- Competitive positioning: Market share by region, pricing analysis (USD/test), partnership heatmaps
Download a Free Sample Report:
https://introspectivemarketresearch.com/request/20209
About Introspective Market Research
Introspective Market Research(IMR) is a globally trusted provider of strategic intelligence across life sciences, diagnostics, vaccines, and global health security. Our team of epidemiologists, regulatory strategists, and commercial analysts leverages primary data from 400+ healthcare providers, labs, and ministries-combined with real-world outbreak analytics-to deliver foresight that informs policy, investment, and product development.
We don’t track trends-we help shape resilient health systems.
Media Contact
Aditi Mehta
Senior Director, Global Health Communications
Introspective Market Research.
Contact Us:- +91 91753-37569.
info@introspectivemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


